Rank the Following From Most Acidic to Least Acidic.
38a solve the problem correctly please and no need to explain too much. Solve Study Textbooks Guides.
Solved Rank The Following From Most To Least Acidic Rank Chegg Com
JOH circle the reducing agent s 720 NaoH Na BH 4 LIATH 4 Fh.

. I II III IV VO H CH3 H HH H NH HCOO H Answer. Memorize flashcards and build a practice test to quiz yourself before your exam. 1 CH3CH2OH 2 CH3CH2OCH2CH3 3 CH3CH2NH2 Select one.
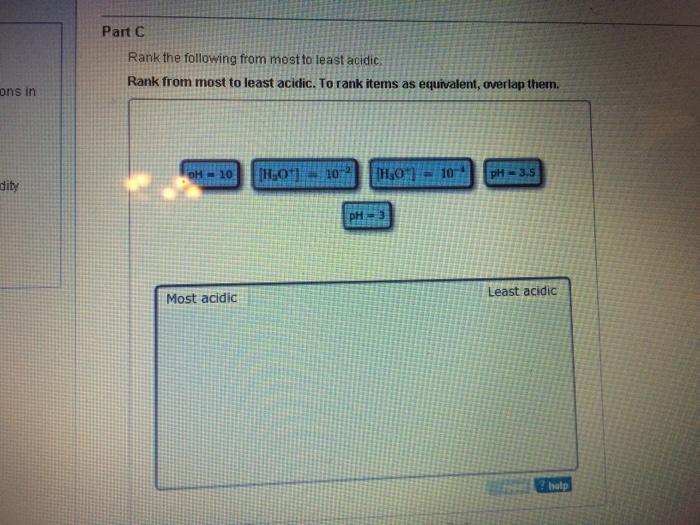
Most to least H3O410-2 pH25 pH4 H3O410-5 pH9. Rank the following from most to least acidic. The remaining solution was acidic.
3 1 2 c. Carbonated cola is more acidic than coffee or even orange juice because cola contains phosphoric acid. Solution for Rank the following from most acidic to least acidic Strongest acid HCI E CH3COOH CH3OH NH4 Fo NH3 Weakest acid.
B it can dissolve in water. B a d c. H3o10-2 pH3 pH10 H3O10-4 pH35.
Rank the following alcohols from least to most acidic. Rank the following from most acidic to least acidic. Rank the following compounds in order from most acidic to least acidic.
Since presence of NO 2. This question asked us to rank the indicated hydrogen is in each of these compounds from most acidic to least acidic. Over Short Answer 2.
D it is a proton acceptor Which acid-base definition classifies an acid as an electron-pair acceptor. Hence 2 will be least acidic. To rank items as equivalent overlap them.
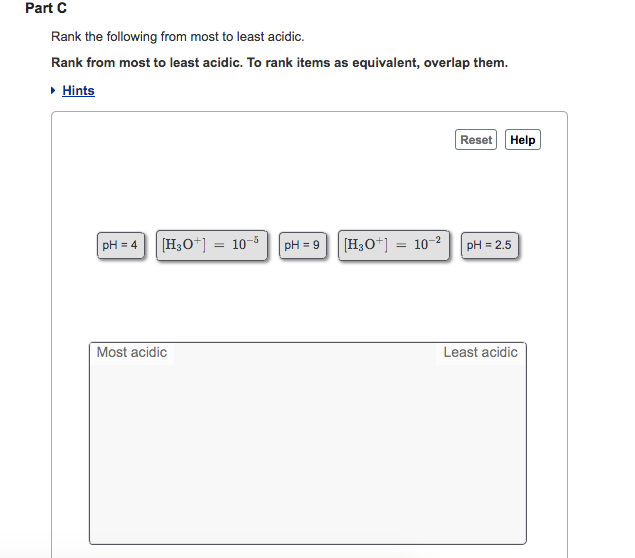
Rank from most to least acidic. Part C The relationship between pH and acidity. Chemistry questions and answers.
What mass of pure Ba OH2 was present. 3 2 1 b. 1 3 2 d.
Start your trial now. Rank from most to least acidic. C d a b.
III I V II IV 42 Rank thebold-faced hydrogens for the following compounds from most acidic to leastacidic. Group in para position causes more electron-withdrawing effect than that in meta position 1 will be less acidic than 3. II V III I IV 43.
Rank the following from most to least acidic. B E OB. It was then titrated to equivalence point with 1200 mL of 05000 M of NaOH solution.
And we can judge these hydrogen acidity based on the stability of their constant basis. 0 758 x 10-4 O 759 x 10-4 O 76 x 10-4 O 3120 x 10-3 0494 O 1318 x 103. Rank the following from most to least acidic.
Label the following diagram of water molecules indicating the location of bonds and the partial charges on the atoms. D a b c. To rank items as equivalent overlap them.
Rank the following compounds by solubility in acidic water from most soluble to least. Find an answer to your question Rank the following from most to least acidic. In m-nitrophenol the inductive effect of the electron-withdrawing nitro group helps to stabilize the.
Hence the order of acid strength is 3142. Rank the following from most to least acidic. So the contact base would be an an eye on if the loan hair on that carbon nestle carbon on and the more residents.
For the following compounds estimate the pKa of the hydrogen indicated by the arrow and rank the compounds from the MOST acidic to the LEAST acidic. 3 points total a. Further presence of electron withdrawing group and its stronger effect stabilizes the phenoxide more.
Chemistry questions and answers. Imperiali PROBLEM SET 7 this really is PS7 and the last one was actually PS6 Due in Friday April 11th at 4 pm 1. 41 Rank thebold-faced hydrogens for the following compounds from most acidic to least acidic.
The relationship between pH and acidity Rank the following from most acidic to least acidic. View Available Hint s Reset Help H3O HO 10-pH 35lpH 10 H3O 10-2 pH 3 Most acidic Least acidic. OH OH OH OH H2N NC CI A.
And so we know that resonance will stabilize a base. Help pH 3 pH 14 pH 5 IT H2O4 102 H2O. Rank the following in order of most acidic to least acidic.
A b d c. To rank items as equivalent overlap them. The weakest acid in the table is.
A solid sample of impure Ba OH2 is added to 4000 mL of 05000 M of aqueous HBr a strong acid. Click hereto get an answer to your question Rank the following compounds in order from most acidic to least acidic. Rank from most to least acidic.
I II III IV VCF3 COOH H O H H OH CH3H3CH3C Answer. Solution X pH 850 Solution Y pOH 430 Solution Z OH- 42 x 10-9 M. Start studying the Chemistry Chapter 14 Study flashcards containing study terms like NaOH is a Brønsted-Lowry base because A it is a polar molecule.
Rank the following groups of compounds from most acidic 1 to least acidic 4. Weve got the study and writing resources you need for your assignments. View Available Hint s.
The solutions are ranked from most acidic pH 2 to most basic pH 14. Rainwater has a pH below 7 because raindrops absorb CO2CO2 which can react with water to. Open in App.
Rank these items from most acidic to least acidic. Rank the following molecules from most acidic 1 to least acidic 4 ень сон CH₂CCH CF3 LOH CH₂ CH₂₂-OH 3 3 circle the strongest base 2014 ठस 20¹4 0₂ N 017 04 애 NO₂ circle the molecule with the lowest expected boilling point. First week only 499.
Which of the acids has the weakest conjugate base. To rank items as equivalent overlap them. Rank from most to least acidic.
The 10-6 M H3O solution has a pH of 6 and the 10-2 M H3O solution has a pH of 2. Join Login. C it is a hydroxide donor.
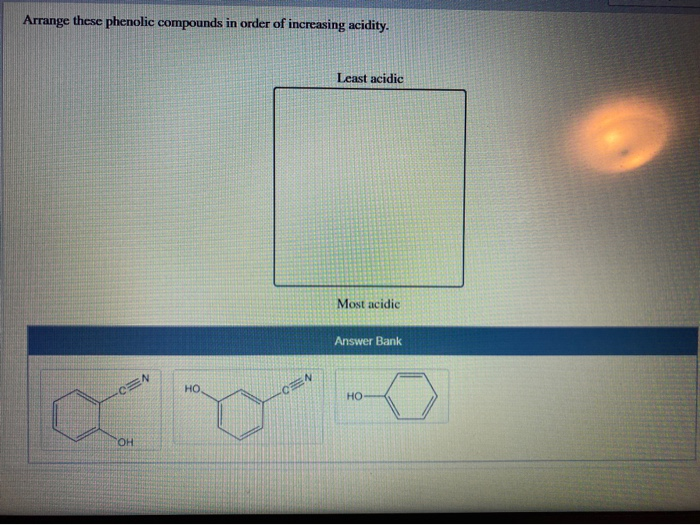
Solved Arrange These Phenolic Compounds In Order Of Chegg Com
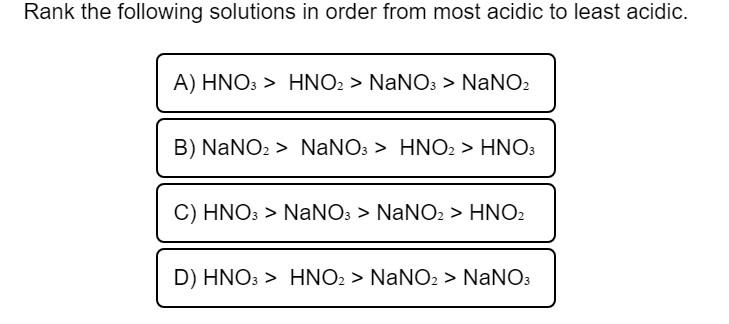
Solved Rank The Following Solutions In Order From Most Chegg Com
Solved Part C Rank The Following From Most To Least Acidic Chegg Com

No comments for "Rank the Following From Most Acidic to Least Acidic."
Post a Comment